Streptococcus Pyogenes - Killer Flesh-eating Bacteria
Created | Updated Apr 14, 2008
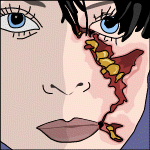
Necrotizing fasciitis, caused by Streptococcus pyogenes the flesh-eating bacteria, begins with a minor injury, usually on an extremity. Wounds of any sort will hurt, but in this case the pain is excruciating. Fever begins to set in, and an unusual redness develops around the area of injury and rapidly moves away from the initial site. By the next day, the redness has turned a strange blue colour, and the skin begins to blister horribly, the bullae1 containing sickly yellowish to bluish fluid. Some of these blisters may haemorrhage, causing an eruption of foul-smelling pus and loss of copious amounts of blood. The pain at this point is severe, almost intolerable. By day four, it is obvious that gangrene has set in, and massive sloughing of the skin occurs. At the hospital, surgeons plunge into a desperate, exhausting battle against the disease, fervently hacking away flesh that becomes rotten almost as fast as scalpels can cut it away. The prognosis is very poor, and the struggle for life continues until death or disfigurement comes - or maybe both.
This is the gruesome tale of a vicious disease that has a preference for human flesh, a pestilence that surpasses the worst of nightmares. However, unlike a nasty Hollywood horror story that keeps you up all night in terrified fascination, this one is made by Mother Nature, and is very, very real.
A Rogue Microbe
The most infamous flesh-eating bacterium is Streptococcus pyogenes2. S pyogenes is a gram-positive round-shaped (coccus) bacterium that tends to grow in pairs and short chains. It is facultatively anaerobic, which means that it can survive in both with and without oxygen (although most facultative anaerobes would greatly prefer aerobic conditions) and requires somewhat complex media for growth. It produces an enzyme called haemolysin, which causes red blood cells to burst. When cultured on blood agar, it produces a clear zone around each colony.
Normally a harmless inhabitant of the mouth and upper respiratory tract, rogue S pyogenes is immensely destructive. It causes, among other things, strep throat (streptococcal pharyngitis), acne, rheumatic fever, toxic shock syndrome and - most horrifyingly - necrotizing fasciitis3.
A Short History of Streptococcus
5th Century BC - Hippocrates describes a scarlet fever epidemic.
1874 - Billroth demonstrates this organism is present in cases of erysipelas4 and wound infection.
1884 - Pasteur is the first to report isolation of this organism from the bloodstream of a woman with puerperal sepsis (sepsis connected with childbirth)5.
Late 19th Century - Rosenbach designates this organism S pyogenes.
1919 - Brown describes the classic differential patterns of alpha (incomplete), beta (complete) and gamma (none) haemolysis6 on blood agar plates.
1930s - Lancefield identifies and describes streptococcal serogroups A - S (a grouping of micro-organisms based on their reaction with serum). S pyogenes is classified a Group A Streptococcus (GAS).
Invasion and Infection
Streptococcus pyogenes' invasion strategy is somewhat direct - and brutal. It usually enters the human body where there is a break in the skin caused by injury. This includes cuts, burns, penetrating injuries, blunt trauma operative sites, enterostomy (the surgical operation of making an artificial opening into the intestine through the abdominal wall, as for drainage), ulcers and diabetic feet. Once in the bloodstream, the bacteria moves rapidly through tissue in a path along the fascia, which is the subcutaneous tissue (just beneath the skin) surrounding the muscle. Rapid multiplication of this invading army causes antibodies to recognise them and to release chemicals called cytokines7 that signal white blood cells to swallow the bacteria.
In the case of S pyogenes infection, phagocytosis (ingestion) of the bacteria is not necessarily a good thing - they pose a 'digestive problem' for the macrophages. An important cell wall component called peptidoglycan is very resistant to digestion, and will persist in the macrophages for as long as 146 days! Therefore, macrophages that have 'indigestion' (ie are laden with too much peptidoglycan from one too many killings) will accumulate in sites of infection. These cells are leaky, and enzymes that leak out from it will cause, among other things, local damage to collagen fibres and the connective tissue matrix. Many of these macrophages will die or form giant cells, and cause chronic inflammatory lesions in the host.
However, all pathogenic strains of S pyogenes have a protein called M protein, which enables them to escape from being eaten. The M protein prevents opsonization (the coating of a particle surface to facilitate engulfment by phagocytes) of the bacteria by a component of the complement cascade called C3b. This prevents formation of the enzyme C3 convertase, thus stopping the classical pathway cascade. Thankfully for us, S pyogenes isn't completely able to avoid phagocytosis. This is because when the bacterial cell breaks apart, it releases certain components that can also activate the complement cascade. At the same time, S pyogenes releases a torrent of toxins that cause massive tissue damage. This extensive tissue damage manifests itself as necrotizing fasciitis.
If the disease is allowed to progress, the body can go into systemic shock, resulting in multiple organ failure, collapse of the circulatory system due to disseminated intravascular coagulation (clotting of blood, spread out within the blood vessels). The coagulation cascade is activated during septic shock, causing formation of numerous small blood clots, which obstruct normal blood flow. The body's blood supply is consequently sequestered in the small blood vessels instead of the major ones, resulting in a drastic drop of blood pressure. The decrease of blood flow to essential organs in the body leads to oxygen starvation and, eventually, organ failure. Because this consumes abnormal amounts of fibrinogens and platelets (essential components in blood coagulation) these resources are depleted, resulting in haemorrhages that can further damage organs. Death is imminent.
The Warning Signs
The symptoms of necrotizing fasciitis caused by Streptococcus pyogenes includes:
- Trauma (day zero)
- Discomfort in the general region of the trauma (day zero).
- Pain that is out of proportion with the severity of the injury, or pain that is not restricted to the immediate area of the injury.
- Influenza-like symptoms such as vomiting, diarrhoea, dehydration, malaise, weakness, muscle pain, and fever
- Swelling or sunburn-type redness in the general region of the injured area (day two).
- Worsening of the condition.
- Less frequent urination.
- Large, boil-like blisters (bullae) containing foul-smelling pus (day two to day three).
- Haemorrhage from the bullae.
- Gangrene (day four)
The War against the Invaders
Early diagnosis of necrotizing fasciitis increases the chances of the victim surviving this disease. Once detected, rapid treatment must begin in order to both save the patient's life and to reduce damage to the patient. However, even with surgery and antibiotics therapy, the mortality rate is high - especially if the patient develops septic shock. Treatment will include:
Aggressive combination regime antibiotics therapy - A broad spectrum of drugs will be used, including cefazolin, clindamycin, gentamycin, penicillin8 and metronidazole. However, because antibiotics therapy sometimes does not work, doctors are pressured to either prevent shock from happening at all, or to catch it in the early - and more easily treatable - stages. If the bacteria in the bloodstream have reached dangerously high levels, antibiotics therapy can actually do the patient harm rather than good. Therefore, scientists have been working to develop drugs to stop the cascade of events that cause damage to the blood vessels and organs.
Theoretically, antibiotics therapy combined with anti-inflammatory drugs should be effective in treating all but the worst of septic shock cases; however, these anti-inflammatory drugs may actually worsen the patient's condition because they further suppress the patient's immune system and increase the risk of secondary infections. Monoclonal antibodies against LPS (lipopolysaccharide - which causes septic shock) have been raised and tested, but therapy using these antibodies must be carried out very early in the shock process to have any positive effect. Other therapies that have been developed - those directed against TNF9 and other shock-related cytokines - are too expensive (at least $2500 per treatment course) to be of any practical value.
Surgical debridement - ie cutting away dead or infected tissue, or removal of foreign matter from the flesh. This involves surgical excision and drainage to remove all infected, necrotic tissue and fascia until clean, healthy, pearly grey fascia is identified in all margins of the wound. This will be carried out as soon as the patient is diagnosed with necrotizing fasciitis to prevent further spreading of the disease. If diagnosed early, tissue loss can be relatively small - only flesh, subcutaneous tissue and fat will be removed. If the disease is allowed to progress, however, amputation of affected limbs may be necessary if the victim is to survive.
Treatment may also include usage of a hyperbaric10 oxygen chamber - This is to increase the partial pressure of oxygen in the blood, thus enabling wounds to heal better by reducing oedema due to vasoconstriction of the arterioles and hypoxia. The white blood cells are also strengthened in their ability to fight the invading bacteria. However, this treatment is more commonly used in the case of gas gangrene, which is typically caused by Clostridium perfringens.
These are only essential treatment measures; however, because of the complication of necrotizing fasciitis, subsidiary patient management is needed to deal with the excruciating pain experienced by patients during dressing changes, and to encourage the body to heal. The caloric intake of patients recovering from massive trauma is usually increased twofold or threefold, often with dietetic recommendations by a dietician. The emotional trauma suffered by the patients due to pain, severe disfigurement following surgery, and intense emotions must also be handled to ensure complete recovery.
Risks
Since Streptococcus pyogenes is such a common bacteria in our lives, it is entirely fortunate for us that the occurrence of necrotizing fasciitis is so rare. A 1996 CDC (Centre for Disease Control) report estimates from 500 to 1500 cases per year of necrotizing fasciitis, of which 20% result in death. Most of us will repeatedly come into contact with this bacterium, and in most cases, will experience infection. However, most of these infections tend towards routine strep throat (pharyngitis) or skin infection (impetigo). Only a very small percentage of people will develop so serious a disease as necrotizing fasciitis. Many of the affected are elderly or those who suffer disease of some sort - for example, cardiovascular or renal disease, cancer, diabetes or a severe weight disorder. However, in a 1993 study, it was found that a group of young, healthy individuals came down with the disease, and that in 50% of the cases, there was no identified portal of entry. Children with chickenpox are an especially high-risk group, and those who are below ten are 39 times more likely to develop invasive GAS infections (including necrotizing fasciitis), the attack rate being 44 per 1,000,000 cases of chickenpox.
Recently, however, it has been established that the usage of pain-relieving non-steroidal anti-inflammatory drugs (such as ibuprofen) greatly increases the risk of developing necrotizing fasciitis, especially among children with varicella-zoster virus infection (aka chickenpox). Because these drugs prevent inflammation, which is important for fighting microbes, it also messes up the body's defence mechanism, thus causing multiple organ system failure. Under normal circumstances, when a macrophage responds to bacteria, cytokines called tumour necrosis factor (TNF) are released into the bloodstream. TNF causes activation of neutrophils (a type of white blood cell), inflammation and fever, and initiates breakdown of muscle and fat. Upon reaching the brain, TNF signals the brain to produce prostaglandins, which will subsequently cause fever. This eventually turns off TNF production. However, non-steroidal anti-inflammatory drugs inhibit the production of prostaglandins, thus preventing onset of fever. Overproduction of TNF therefore allows the bacteria to spread more easily, and contributes to shock and organ failure. It has been shown that TNF can cause damage to endothelial cells and provoke abnormal function of endothelial tissue, eg inappropriate constriction or relaxation, eventually causing multiple organ system failure.
Prospects for Vaccines
Although many people get excited at the prospect of vaccines for infectious diseases of any sort, the fact is that a streptococcal vaccine would have very little practical value. This is because there are so many different serotypes of S pyogenes; any vaccine developed would have to span all of these to be effective in combating the disease. Multivalent vaccines containing multiple M-protein peptide epitopes have been engineered, and have been shown to work in animal models; however, there have not yet been clinical trials for these vaccines, and it might be a long time before there are. Furthermore, there is reason for the medical community to be concerned that vaccine-induced antibodies could injure host tissue and cause rheumatic fever. Since the M-protein shares certain amino acid sequence with some human tissues it may cause cross-reactivity between the two, thus causing the body's immune system to attack the body itself.
What You can do to Help Protect Yourself against Necrotizing Fasciitis
There is very little that you can do by way of prophylaxis (control - in this case medication or vaccination) to prevent infection and intra-family spread; however, very little is still more than nothing at all. The most important preventive measure is to keep your skin intact. If your skin is broken by injury, clean the wound thoroughly with disinfectant and cover it up with clean dressing so that it does not become exposed to the environment. Wound dressings should be changed regularly, and the wounds should be monitored for possible signs of infection (see The Warning Signs, above). If you should develop the symptoms listed, immediately seek medical help. It may be disgustingly fascinating to watch the disease run its full course, but it could also cost you your life. If your child has chickenpox, avoid giving them non-steroidal anti-inflammatory drugs to ease the pain. Athletes should also be cautious about using the said drugs.
Do not panic unnecessarily. You should, of course, exert caution, but you should also realise that the chances of you developing necrotizing fasciitis in your lifetime (if you are a healthy individual) are very, very small.
Pathogenicity of Streptococcus Pyogenes
Virulence Factors of Streptococcus Pyogenes
Streptococcus pyogenes possesses a number of somatic (non-gametic) cellular constituents as well as extracellular (outside of the cell) enzymes and toxins that are responsible for its pathogenicity (ability to cause disease). These include:
M-protein - The ability of S pyogenes to cause disease greatly depends upon this protein. It is anchored to the bacteria's cell membrane. It serves to inhibit the activation of the complement cascade11 and protects the bacteria from being gobbled up by phagocytes (white blood cells that engulf and kill invading bacteria - macrophages are a type of phagocyte). There are 80 different types of this protein. Antibodies are generated against the M protein and help to destroy it, as well as to protect from infection against other S pyogenes that possess the same M protein; however, they are useless against other M types. Non-pathogenic strains usually do not have this protein.
Hyaluronic acid capsule - Most strains of S pyogenes are enveloped in this. It protects the bacteria from being phagocytosed. Hyaluronidase is an enzyme that liquefies the hyaluronic acid (a component of connective tissue) component of the connective tissue matrix.
Lipoteichoic acid12 and protein F - These enable the bacteria to stick to fibronectin (an adhesive glycoprotein that helps destroy bacteria, etc, in the blood or that helps structure connective tissue) on the surface of human epithelial cells13. This is crucial if the bacterium is to succeed in its invasion.
Streptolysin - These enzymes are responsible for the bacteria's haemolytic activity. There are two types of streptolysin:
Streptolysin O - This enzyme is sensitive to oxygen and can be inactivated by it. It not only destroys both red and white blood cells, but also provokes an immune response (ie it is antigenic). It is toxic to a wide variety of cells, including the myocardium (the muscular substance of the heart).
Streptolysin S - This enzyme is oxygen-stable. It is also responsible for beta-haemolysis, but is not antigenic.
A large number of other toxins and extracellular products also play a role in tissue damage and the spread of S pyogenes; however, they will not be discussed here.
The Superantigen Phenomenon
Usually when the immune system encounters an antigen, this provokes typical antigen-specific interference, such as phagocytosis. However, there are certain types of antigens that cause a different sort of response. These are called superantigens, and S pyogenes is one of the bacteria that produce this type of antigen.
Under normal circumstances, antigen presenting cells (APCs) - such as macrophages that have engulfed bacteria - process protein antigens by cutting them up, and presenting one of the pieces on the cell surface. Only a few T cells will be able to recognise these pieces, and therefore only a few will be stimulated to produce cytokines that lead to antibody production (about 1 in 10,000 T cells).
Superantigens, however, are not chopped up, but will bind directly to the APC's surface. Because they do this indiscriminately, many APCs will have these superantigen molecules bound to their surfaces. Also, these superantigens bind indiscriminately to T cells as well, and therefore will stimulate as many as one in five T cells. This causes release of excessively high levels of the cytokine IL-2, thus giving rise to a variety of symptoms such as nausea, vomiting, malaise and fever. This also leads to excess production of other cytokines, which will subsequently cause shock.
Bibliography
Davies HD, A McGeer, B Schwartz, K Green, D Cann, AE Simor, DE Low, The Ontario Group A Streptococcal Study Group, 1996. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med; 335:547-554.
Fink, A and G DeLuca. 2002. Necrotizing fasciitis: pathophysiology and treatment. Medsurg Nursing. 11:33-36.
Foth, J, 2002. Necrotizing Fasciitis: 'The Flesh-Eating Bacteria'. Science Education.
Gladwin, M and B Trattler, 1997. Clinical Microbiology Made Ridiculously Simple. McGraw Hill International Editions.
Mims, CA, NJ Dimmock, AA Nash and J Stephen (Editors), 1995. Pathogenesis of Infectious Disease, 4th edition. Academic Press Limited, London.
Salyers, AA and DD Whitt, 1994. Bacterial Pathogenesis: A Molecular Approach. ASM Press, Washington DC.
Schleiss, MR, 2002. Streptococcal infection, Group A. EMedicine.
Seachrist, L, 1995. The once and future scourge. Science News. 148:234-5.
Stevens, DL et al, 1989. Severe Group A Streptococcal Infections Associated with a Toxic Shock-like Syndrome and Scarlet Fever Toxin. NEJM 321:2-7.
Webster's New World Dictionary, Third College Edition (1994).
A lot of information can also be found at the National Necrotizing Fasciitis Foundation.
To read a story about a survivor's battle against necrotizing fasciitis, go to A Survivor's Battle.

