Crystals, X-rays and Diffraction.
Created | Updated Jan 28, 2002
Crystals can be very beautiful, but there are a lot of misconceptions about them. X-rays can be very dangerous, but there are also a lot said about them that is apocryphal. More importantly however, X-rays can be used to see inside a crystal in a unique way.
Crystals!
People always think of crystals as pretty little, gem-like things, that have nice sharp edges and a symmetric shape. This is often the case but not always
1
. A crystal is defined as
"...a small piece of a substance that has formed naturally into a regular symmetrical shape."(Collins Cobuild English Dictionary)
But the Oxford Concise Science Dictionary goes on to add:
"...The atoms, ions, or molecules forming the crystal have a regular arrangement..."
which is the important point that scientifically defines a crystal.
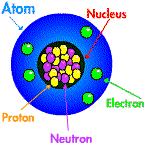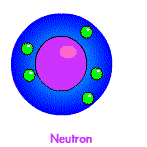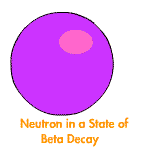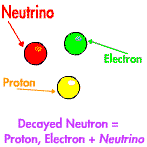
So scientifically, a crystal is a collection of ordered atoms, but what does this actually mean? Well, if you imagine packing red and blue cubes into a box, you would start in a corner and pack along an edge. You might start with red, blue, red, blue etc. If * represents red and $ represents blue, you would have:
* $ * $ * $ * $ * $ * $
The next row might start blue, red etc. and the third red, blue etc. building up a pattern
* $ * $ * $ * $ * $ * $
$ * $ * $ * $ * $ * $ *
* $ * $ * $ * $ * $ * $
$ * $ * $ * $ * $ * $ *
This is a lattice and if you continue with the second, third etc. layer in the box you get salt. Not exactly salt you understand, but a pretty good representation of it. That is what they teach in schools; salt is made up of sodium and chlorine - sodium chloride - and the ions alternate. In fact actual atoms or ions are spherical, not cubes and sodium is smaller than chlorine, but the idea's the same.
X-rays!
X-rays are just a form of light. Light travels in waves:
o o$ o$ oo
oo oo oo oo oo oo oo
o o o o o o o
o o o o o o o
o o o o o o o
o o o o o o o
o o o o o o o
o o o o o o o
oo oo oo oo oo oo oo
oo oo oo etc....
These waves have a frequency and a wavelength, which defines the type of light it is. From the diagram, it can be seen that the waves repeat - if you start at the top of any hump ($), exactly the same happens until you get to the top of the next hump ($). The wavelength is the distance from the top of one hump to the top of the next. This is of course the same as the distance from the bottom of any pit to the bottom of the next (and likewise form any other point to the next identical point). The frequency is related to the wavelength by an equation:
c=fλ
where c is the speed of light, f the frequency and λ the wavelength. Since c is a constant
2
this can be written as:
f∝1/λ
which means, that as the wavelength gets larger, the frequency gets smaller. This makes sense because if you think about it, the larger the wavelength, the less 'squashed up' things are.
So, we have light which has different frequencies and different wavelengths. This is called the electromagnetic spectrum and it consists of many types of waves, from microwaves (what you cook dinner with), to radio waves (what you listen to Hitchikers' Guide to the Galaxy with), to visible light (what you see things with), and X-rays (what we look ar crystals with).
Diffraction!
X-rays have a wave length of approximately 1Å, that's about one billionth of a meter! Coincidently, this is approximately the same as the distance between atoms. This is very important as it is what enables us to 'see' what goes on inside crystals.
OK, so imagine you drop a stone into a pond. The ripples radiate in circles from where the pebble hit the water. OK, so imagine you drop two stones into the pond - there are two lots of circles. These circles are waves; they have peaks and pits. Now comes the hard bit - what happens where the circles meet?
Well, it's called interference. So, where two peaks meet, there is a peak twice as high, and where there two pits, there is a pit twice as deep. That's constructive interference. Now comes the difficult bit; what happens when a peak meets a pit?
Well, the peak and the pit cancel out and you get nothing, this is called destructive interference.
Now comes the really difficult bit. What if many stones were dropped at the same time? Well, the pattern is very complicated, but the same rules apply. Where only peaks meet, they will add up and where only pits meet, they 'add down', but where a combination of peaks and pits meet, the effect depend on how many of each.
ie. 3 peaks + 2 pits = 1 peak
So, what has this to do with crystals and X-rays? Well, this is a very good analogy for diffraction. When X-rays shine at an atom, they effectively behave like the waves leaving the stone in the pond, except that they leave in all three dimensions, so that's above and below too. Of course there are many atoms in a crystal, so there is interference on a grand scale! This interference is used by crystallographers to study what goes on inside a crystal.
Crystallography!
Crystallography is the study of crystals. It uses diffraction
3
to determine what the structure is. Crystallographers use machines called diffractometers which basically shine X-rays at a crystal sample. The diffraction is then detected by a sort of camera which records a pattern.
Some complicated maths and some fancy computer programmes then use this pattern to suggest a structure, which is then modified. This new structure is then used to generate a pattern, which is then compared with the original data. The differences between the two patterns are then used to up-date the structure and improve it. This is called refinement.
Once the structure has been fully refined a picture can be produced. . Crystallography is the only way this can be done and is an extremely valuable tool used in pharmaceuticals and pictures of some readily available household drugs have been obtained this way, for example aspirin, paracetamol and ibuprofen. Crystallography can tell you the distances and angles between atome in a way that no other technique can and as such it is one of the most powerful analytical techniques used in science. However, the material must be crystalline, that is to say it MUST have long range order and this is not the case for all solids.
Shattered glass can have a symmetric shape and sharp edges for instance, but is not crystalline.
2
Light travels at the same speed regardless of it's frequency or wavelength. However, the speed of light is dependent on what it is travelling through. This is why when you put you finger in to water it looks like it doesn't join up properly. Imagine a column of soldiers walking into a swamp; when each soldier reaches it, they will slow down, but they will slow down one at a time. Now imagine them walking at an angle, the first row is shown here as $, the rest of the column, behind them as * (veiwed from above). As the first one slows down, the rest are left walking faster and so the column changes angle as it enters the swamp (the reverse happens as they leave the swamp). This is sort of what happens with light; it bends - this is called refraction.
*
* *
* * *
* * * *
* * * * *
* * * * *
* * * * *
* * * * $
* * * $ .
* * $ . .
* $ . . .
$ . . . .
===================================================
. . . . .
. . . . .
*Water* . . . . .
. . . . .
. . . . .
. . . . .
===================================================
. . . . .
. . . . .
. . . . .
. . . . .
. . . .
. . .
. .
.
Most commonly, X-rays are used, but neutrons can also be used. Neutron are small particles found in the centre of atoms, the nucleus. They can be used because they also behave like waves, called wave-particle duality - Wierd!. The most common source of neutrons is a nuclear reaction, but there are other ways of making them. Since they are quite dangerous there a very few places that do neutron diffraction and these are large scale reasearch facilities, eg. ISIS, ILL and IPNS.
You can also use synchrotron radiation, which is really just faster, harder X-rays that have been generated by accelerating electrons round a synchrotron. This is also only done at large scale research facilities, eg. The Daresbury Laboratory, ESRF and NSLS